Nanochemical Imaging
We have performed lateral force microscopy experiments, both for nanochemical imaging of surfaces and for fundamental studies in tribology.
Conventional techniques for the chemical imaging of organic and inorganic surfaces, such as iXPS or iToF-SIMS, are limited in their spatial resolution to a tenth of a micrometer. Scanning probe techniques, on the other hand, allow a spatial resolution down to nanometer scale, but do not inherently give information about chemical properties. By the modification of probes or by changing the medium of measurement, however, specific chemical properties of surfaces can be probed with nanometer resolution.
The imaging of oxide as well as organic surfaces with submicrometer resolution is an urgent need in many technological areas. Oxide surfaces play an important role in biomedical applications, for example on implant surfaces. Organic surfaces are used for lubricants, corrosion inhibitors or in the area of biosensors. The distinction between chemically different species is of utmost importance for an understanding of surface properties, e.g. for the adsorption of proteins in biomedical applications, and hence for further future applications.
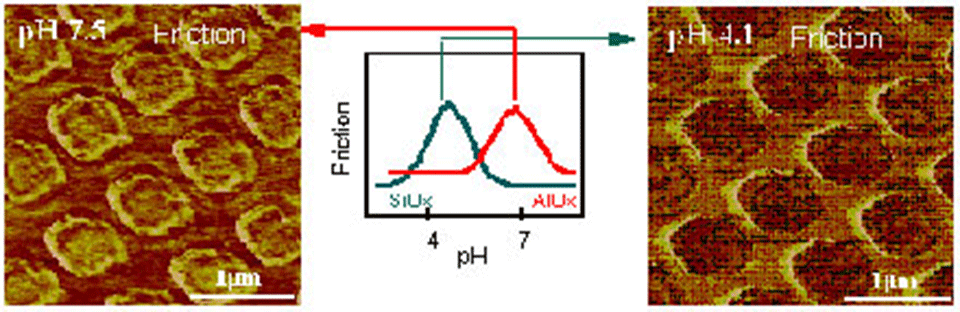
We have investigated the pH-dependence of lateral forces on different oxide surfaces on a nanometer scale with a lateral force microscope (see Digital Instruments) in electrolyte solution. Due to the reaction of hydroxyl groups at the probe and the substrate surface with ions in the solution, the distinguishing of different oxide species with submicrometer resolution is possible. In combination with other surface analytical techniques an identification can be accomplished [1-4] (Figure 1).
A similar approach is applicable on organic surfaces if there are groups present that can deprotonate [5]. However, care has to be taken since the mechanical stability of ultrathin organic films also contributes to the lateral force signal and can be different for chemically identical species [5-7]. The distinction between different polymers in polymer blends can also be achieved by changing the medium or the chemistry of the tip [5]. In this case, not only electrostatic forces, but also van der Waals‘ interactions play an important role (Figure 2).
Although the friction and wear behavior of a large number of material combinations have been studied for many years by classical tribological test methods (e.g. pin-on-disk tribometers), the understanding of the molecular aspects of friction and of the surface-chemical reactions taking place during relative movements of two bodies in contact is still very rudimentary. Lateral force measurements with a scanning probe microscope have the potential to close the gap between the classical macroscopic tribostudies and the urgently needed molecular, nanoscale understanding of surface tribology and chemistry [8,9].
However, in order to understand the mechanisms that cause friction, concepts other than the classical single friction coefficient value are necessary in order to get more insight into the underlying (local) mechanisms that cause energy dissipation while sliding.
Recently we have employed a correlation analysis to study friction traces recorded with a lateral force microscope, since it is there that we can capitalize on the very local information contained in the signal. The autocorrelation provides information on the rate at which the friction force is changing with distance and this, in turn, can give information about the mechanism that causes the friction. The autocorrelation function can be used to deduce junction size assuming that the autocorrelation at constant load becomes zero when the sliding distance becomes great enough so that a ‘new set of junctions’ is involved [E. Rabinowicz, J. Appl. Phys. 27(2), 131 (1956)]. The fluctuations in such traces are due to chemical inhomogeneities or topographical features at the surface. In the case of ultraflat surfaces, however, they are determined solely by the chemical properties of the contact interface between the two contacting bodies.
Our experimental studies in combination with model calculations have revealed that the autocorrelation length, i.e. the distance where the autocorrelation becomes zero, can be used to extract direct information on the nominal contact diameter, and hence on the nominal contact area. In addition, information on the chemical homogeneity of the contact established between the probe and the surface can be deduced from the overall shape and the smoothness of the autocorrelation function.
Publications
[1] A. Marti, G. Hähner, N.D. Spencer; "The Sensitivity of Frictional Forces to pH on a Nanometer Scale - A Lateral Force Microscopy Study"; external pageLangmuir; 1995; 11 pp 4632-4635call_made
[2] A. Marti, G. Hähner, N.D. Spencer; "Reibung und pH-Wert: Eine rasterkraftmikroskopische Studie"; Berichtsband zum Symposium "Reibung und Verschleiß" der Deutschen Gesellschaft für Materialforschung, Bad Nauheim, 21.-22.3.1996
[3] G. Hähner, A. Marti, N.D. Spencer; "The Influence of pH on Friction between Oxide Surfaces in Electrolytes, Studied with Lateral Force Microscopy: Application as a Nanochemical Imaging Technique"; Tribology Letters; 1997; 3(4) pp 359-365
[4] C.E. Sittig, G. Hähner, A. Marti, M. Textor, N.D. Spencer, R. Hauert; "The Implant Material, Ti6Al7Nb: Surface Microstructure, Composition and Properties"; external pageJ. Mater. Sci.: Mater. in Med.; 1999; 10(4) pp 191-198call_made
[5] K. Feldman, T. Tervoort, P. Smith, N.D. Spencer; „Towards a Force Spectroscopy of Polymer Surfaces"; external pageLangmuir; 1998; 14(2) pp 372-378call_made
[6] K. Feldman, G. Hähner, N.D. Spencer; "Surface Nanochemical Studies of Polymers and other Organic Surfaces by Scanning Force Microscopy"; ((ACS Symposium Ser.; 1999; Series 741 pp 272-283))
[7] D. Fischer, A. Marti, G. Hähner; "Orientation and order in microcontact-printed, self-assembled monolayers of alkanethiols on gold investigated with near edge x-ray absorption fine structure spectroscopy"; external pageJ. Vac. Sci. Technol. A; 1997; 15(4) pp 2173-2180call_made
[8] G. Hähner, and N.D. Spencer; "Rubbing and Scrubbing"; external pagePhysics Today; 1998; 1998(9) pp 22-27call_made
[9] K. Feldman, M. Fritz, G. Hähner, A. Marti, and N.D. Spencer; "Surface Forces, Surface Chemistry and Tribology"; external pageTribology International; 1998; 31(1-3) pp 99-105call_made

![Enlarged view: Figure 2: Height (AFM) and friction (LFM) images of a spin-cast polystyrene:poly(methyl methacrylate) polymer blend [PS:PMMA (1:10 w:w)], obtained with gold-coated (top) and SiOx-coated tips (bottom) under perfluorodecalin.](/research/advanced-surface-analysis/nanochemical-imaging/_jcr_content/par/fullwidthimage_1676526633/image.imageformat.1286.934313279.png)