Lubrication Mechanism of Glycoproteins and Sugars
The project involves gaining a greater understanding of the way in which components of synovial fluid, such as glycoproteins, and biomimetic sugar-based polymer brushes, impact friction. The methodology involves studying adsorption of the macromolecules on hydrophobic/hydrophilic surfaces by means of an interferometric sensor, determining the amount of associated water through a quartz crystal microbalance, as well as coordinated tribological experiments.
Recently, in a collaboration between the ETH Laboratory for Surface Science and Technology, Department of Materials, and the Laboratory for Nanoscale Materials Science at the Empa, it has been shown that the sugars attached to cells and to proteins are able to communicate with other biomolecules across water at distances of several tens of nanometers (1).
It has been demonstrated that the sugar residues (“glycans”) on a glycoprotein can influence the structure of water around them—an effect that is dependent on the spatial orientation of the sugar. This modification of the structure of water influences the behavior of other biomolecules that come into the vicinity of the sugar residues. Such indirect interactions could also play an important role in cell-cell signaling mechanisms.
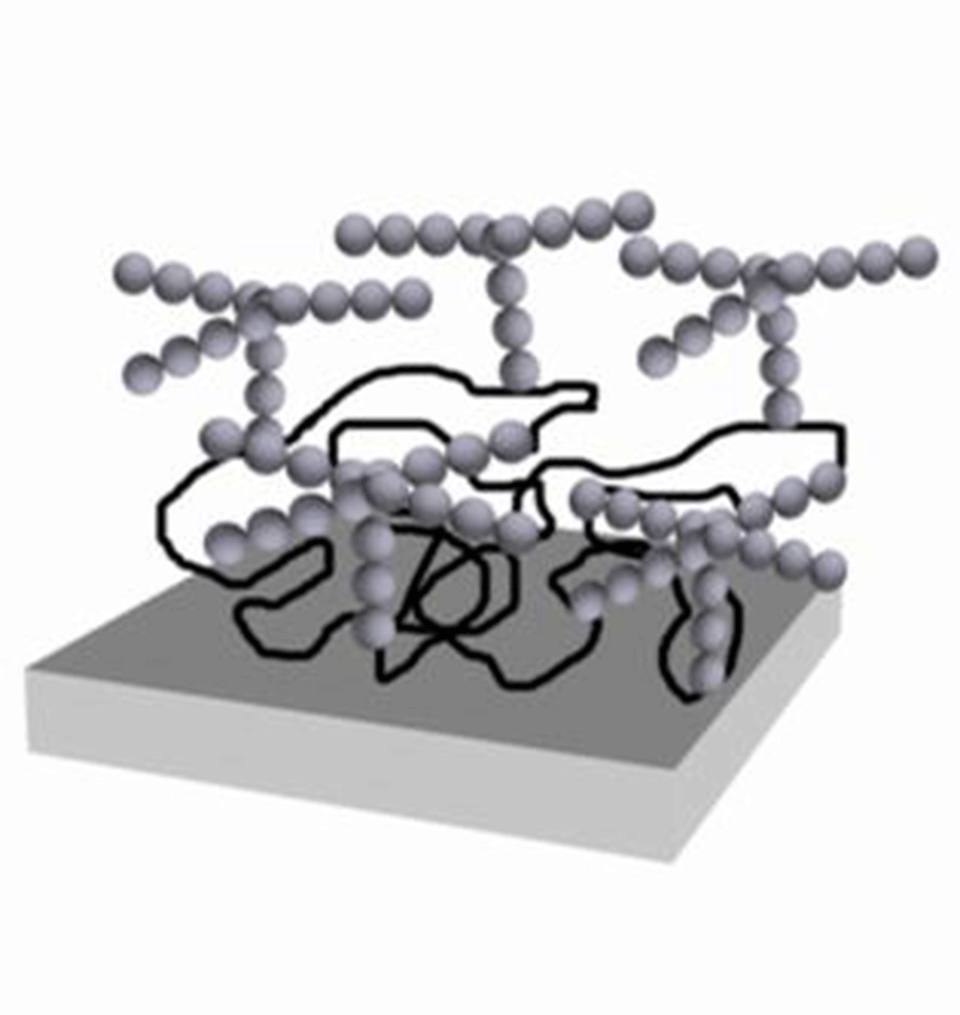
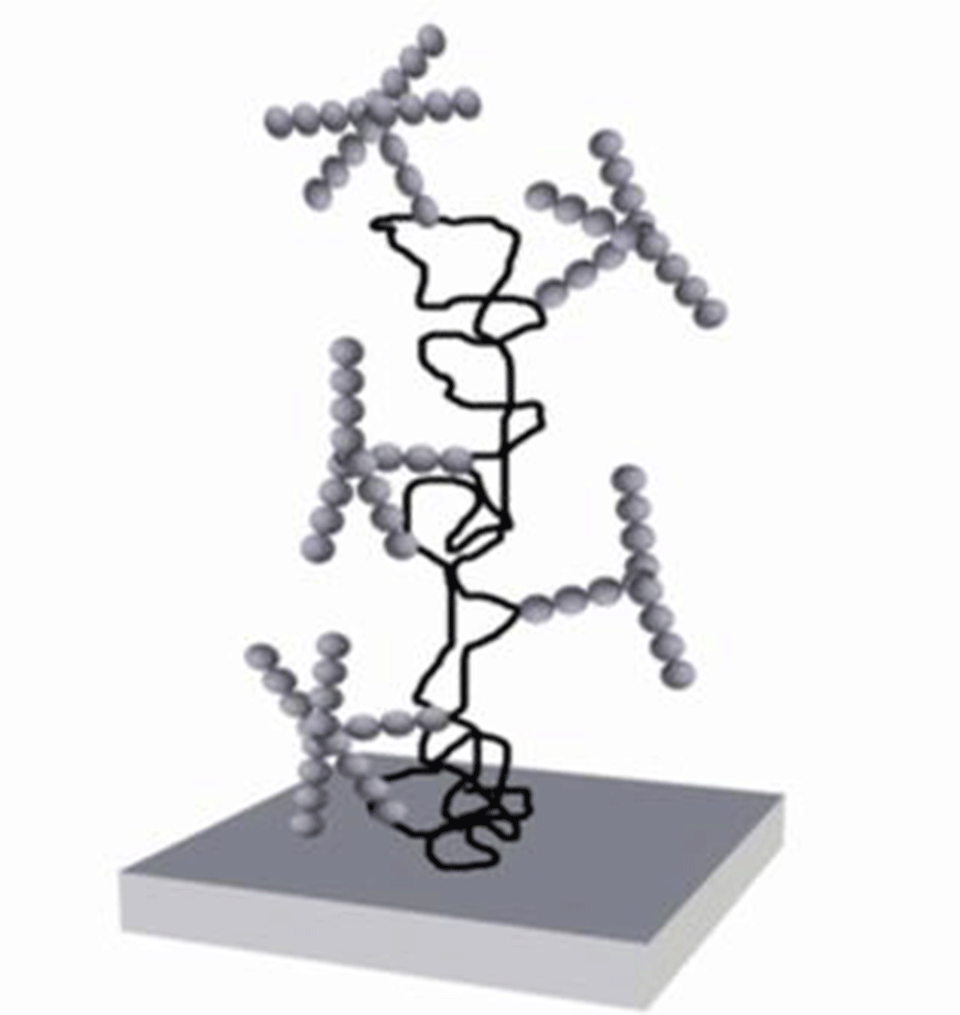
The results were generated using a combination of adsorption and surface-force measurements, the latter indicating a layering of water above a glycoprotein (alpha-1-acid glycoprotein or alpha-1-antitripsin)-covered surface, as it was being increasingly confined by a bare mica surface. The requirement for a specific orientation of the glycans was demonstrated by the disappearance of the layering effect following simple chemical reduction of the glycoprotein. Reduction leads to the unfolding of the protein and therefore a change in sugar orientation (Figure 1).
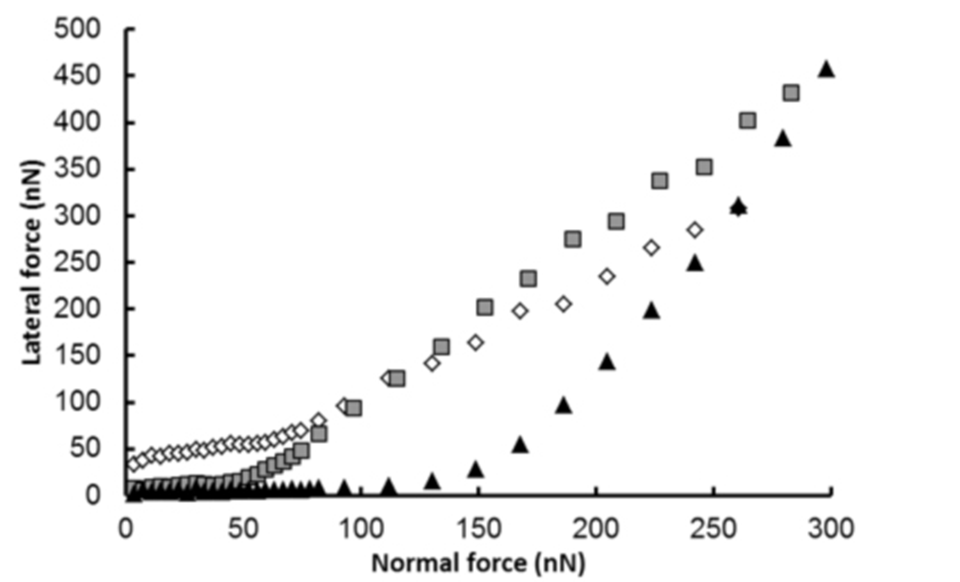
On the other hand, it has been also shown that both the glycoproteins AGP and A1AT, despite their low concentrations in solution, improved lubrication. The lubricating properties of AGP and A1AT were attributed to adsorption via the hydrophobic backbone, allowing the hydrophilic carbohydrate moieties to be exposed to the aqueous solution, thus providing a low-shear-strength fluid film that lubricated the system (2).
Figure 1: Schematic conformation of adsorbed AGP in the absence (a) and presence (b) of the reducing agent. The two disulfide bridges in the native conformation hinder unfolding of the peptide chain. DTT breaks these disulfide bridges, increasing the peptide flexibility. The increased adsorbed mass of reduced AGP does not allow the conformation of the glycans as shown in a).
References
(1) Rosa M. Espinosa-Marzal, Giacomo Fontani, Frieder B. Reusch, Marcella Roba, Nicholas D. Spencer, Rowena Crockett. Sugars communicate through water: oriented glycans induce water structuring, Biophysical Journal (2013), doi: 10.1016/j.bpj.2013.05.017 (in press).
(2) Roba M, Naka M, Gautier E, Spencer ND, Crockett R., The adsorption and lubrication behavior of synovial fluid proteins and glycoproteins on the bearing-surface materials of hip replacements. Biomaterials (2009), doi: 10.1016/j.biomaterials.2008.12.062.
(3) Kenneth J. Rosenberg, Tolga Goren, Rowena Crockett, Nicholas D. Spencer. Load-induced transitions in the lubricity of adsorbed poly(L-lysine)-g-dextran as a function of polysaccharide chain density, ACS Applied Materials and Interfaces; 2011; 3(8) pp 3020-3025