Lubrication with Bioinspired Amphiphilic Polymers
Amphiphilic polymers have shown to be very versatile molecules, because they can interact with each other and adsorb onto surfaces in many different ways, depending on the ionic strength of the solution, the charge density on the polymer and the polymer concentration in solution and the chemistry of the surface. It is thus possible to tune these parameters to obtain polymers that adsorb onto surfaces from aqueous solution and interpenetrate forming a gel-like layer, with the inclusion of a large amount of water. This adlayer has the advantage of lowering the friction between surfaces and can reform itself after friction-induced removal.
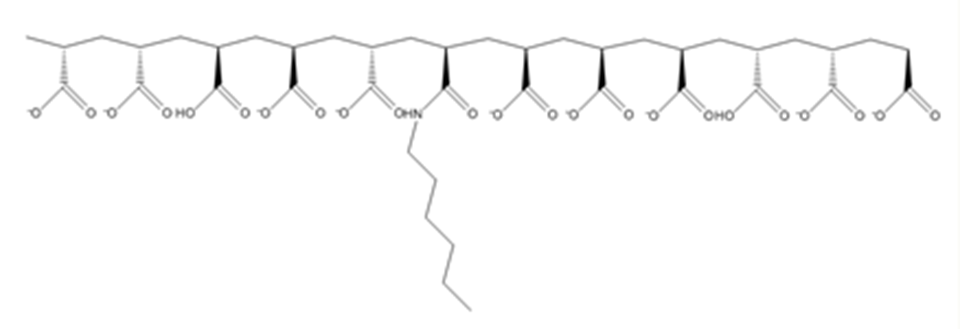
A series of hexyl derivatives of poly(acrylic acid) (HPAA) with differing degrees of substitution were synthesized (Figure 1) and their adsorption behavior from aqueous solutions onto hydrophobic surfaces as well as the friction behavior of the adlayer were evaluated.
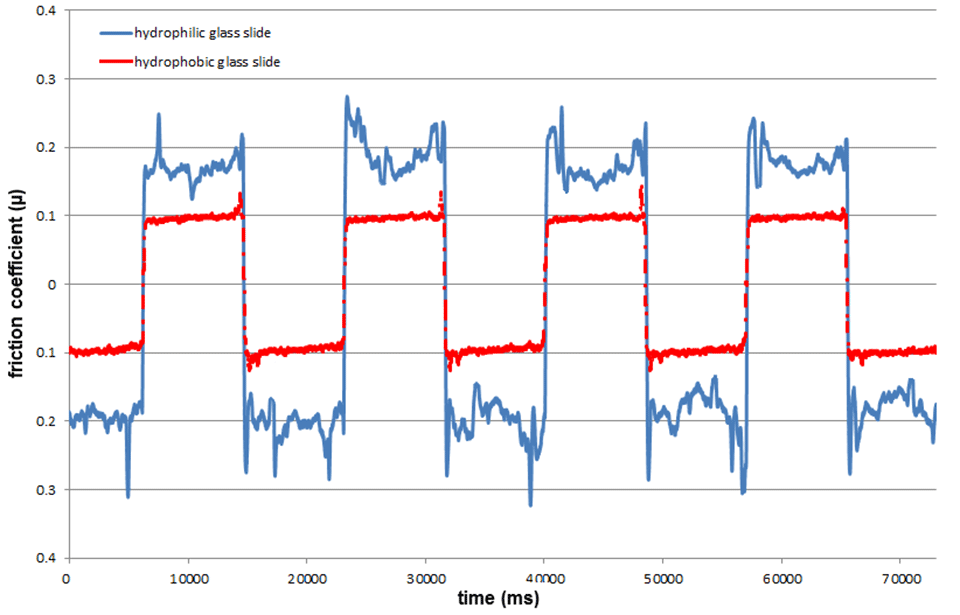
Absorption studies were carried out using the transmission interferometric adsorption sensor (TInAS) and the Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D) techniques, while friction studies were carried out at normal atmospheric conditions on a reciprocating sliding rig. Results show the ability of HPAA to adsorb onto hydrophobic surface, forming a water-enriched layer even at low polymer concentration. This adlayer has the ability to reduce friction between hydrophobic surfaces (Figure 2).
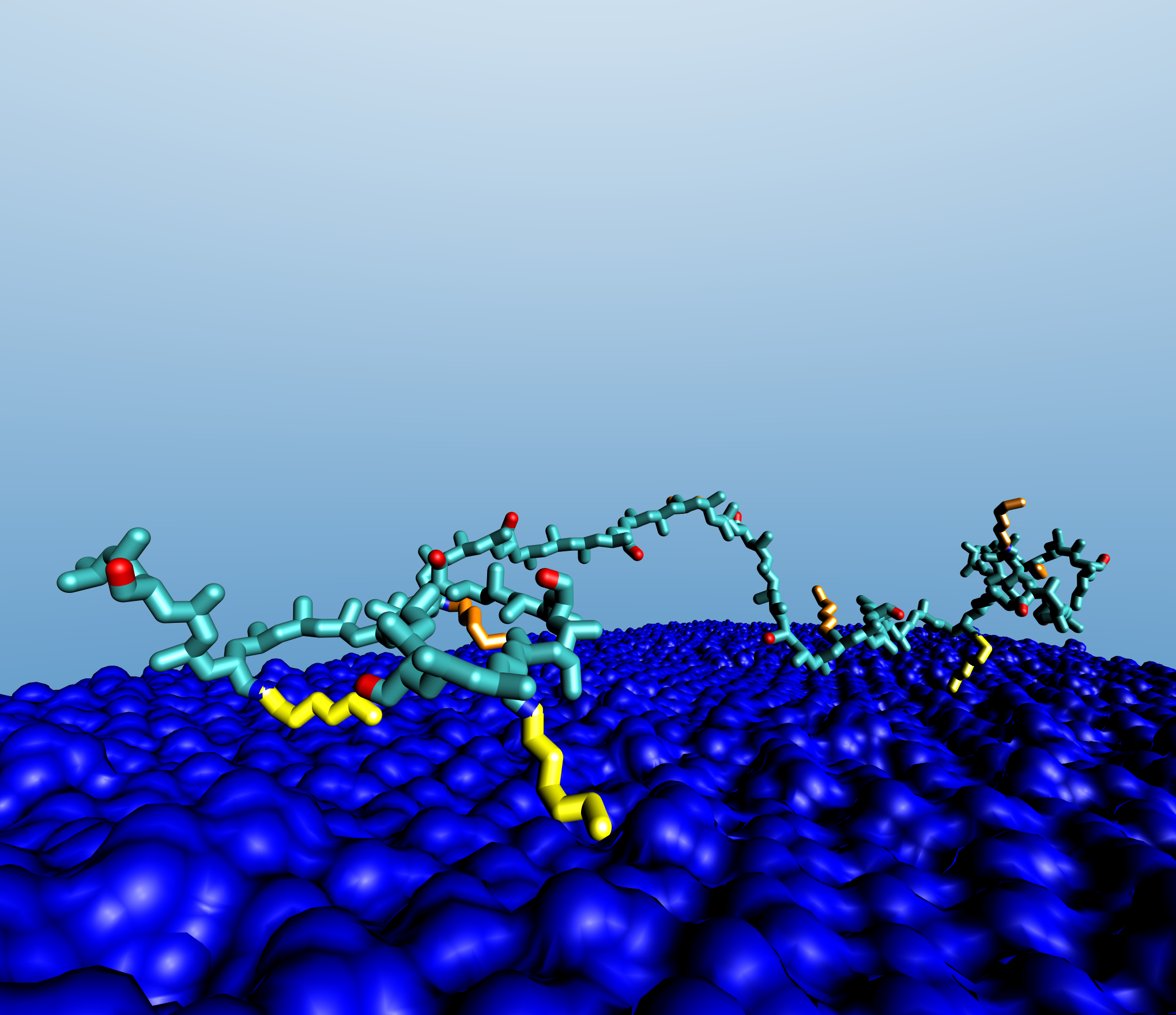
At the same time computational studies were carried out by modeling the polymer at the surface with classical molecular dynamics methods and investigating its behavior once adsorbed onto an octane monolayer, used as a model of the hydrophobic surface. Results suggest that, in presence of water, the polymer undergoes a re-orientation to expose the deprotonated carboxylate groups to bulk water and to direct some of the side chains towards the surface. However, the polymer is not able to re-orientate all of the hydrophobic chains towards the surface and a small number of them remained stretched towards the bulk water (Figure 3).
This conformation is significant as it would indicate that hydrophobic interactions between different HPAA molecules are still feasible after the formation of the first adsorbed layer. A highly ordered multilayer system would not be consistent with the optimized model. Thus, the model suggests that the adsorbed polymer would most probably forms a gel-like layer.