Tribology and Surface Forces
Physics of confined fluids
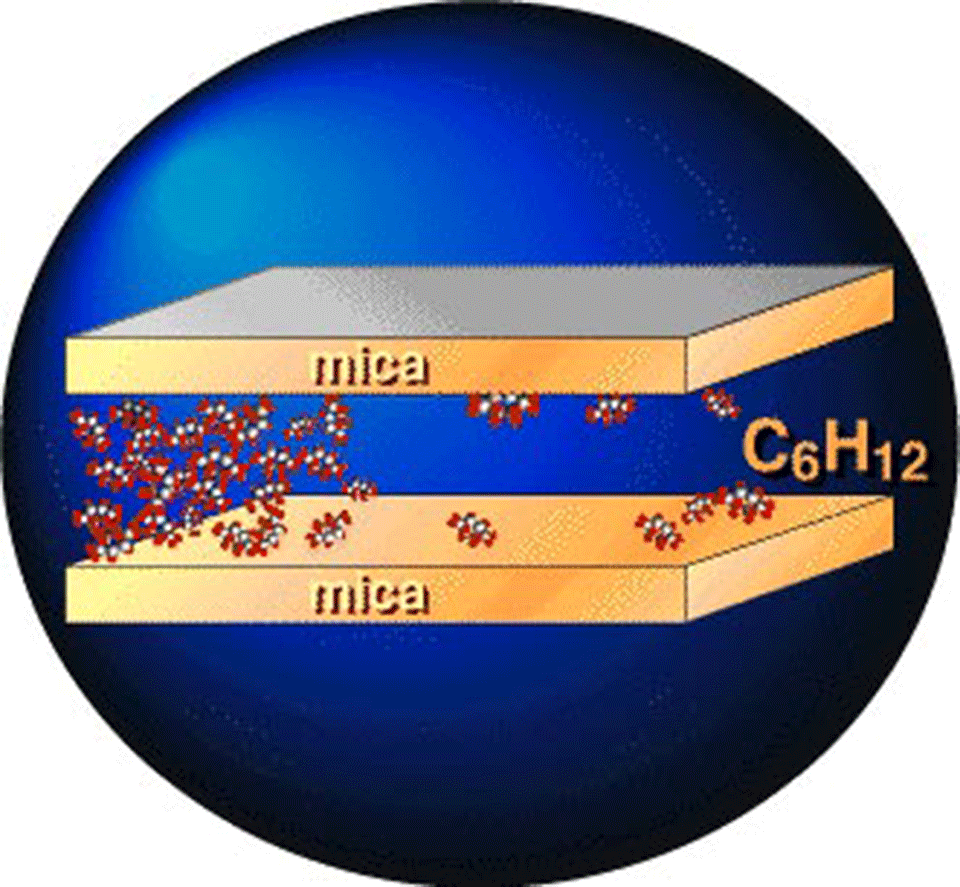
Simple liquids in restricted geometries, such as pores or slits, have been found to exhibit extraordinary properties. Physical properties that are greatly changed by confinement include the effective viscosity, the diffusion coefficient, as well as the melting point. Moreover, neutron scattering experiments and simulations have shown that confinement induces short-range order within these liquids. The crossed-cylinder configuration encountered in most surface forces apparatus (SFA) experiments represents one of many forms of molecular level confinement. It is very special in view of its extremely high aspect ratio; typical values for the surface separation and the contact diameter are 5 nm and 50 µm, respectively, yielding an aspect ratio of 1:10000.
Among the vast array of liquids investigated in the SFA, many exhibit so-called structural forces at surface separations smaller than a few nanometers. The film thickness of confined fluids was found to discontinuously decrease in molecular-sized steps in the last few nanometers of a closing slit. Therefore, the notion “oscillatory force” was created. Its interpretation is based on the assumption that fluid molecules start to order into discrete layers as they become more and more confined. Unfortunately, this picture has remained rather speculative because of the limited information obtained from traditional SFA experiments see Picture 1.
We have developed the extended surface forces apparatus eSFA to measure both the film thickness and the refractive index of a cyclohexane film confined between two smooth mica surfaces. Our force measurements are in agreement with previous cyclohexane experiments. However, due to a more than ten-fold increase in the resolution of our instrument, they also provide new pieces of information about the fine structure of molecular ordering. We find that the refractive index, which is a good measure of the local mass density, exhibits large external pagefluctuationscall_made and its time-averaged value is reduced by about 50% as compared to the bulk liquid value. These density measurements are not easily fitted into the traditional picture of oscillatory forces and therefore necessitate a revised interpretation of the basic physics of fluids under tight confinement. The simultaneous occurrence of fluctuations with solid-like densities and the apparent 'solidification' of the fluid suggest that mechanical properties may be fluctuating locally. The unexpected existence and magnitude of such fluctuations and the related lateral inhomogeneity of the confined fluid represent new pieces of information from a world of tight confinement far away from thermodynamic equilibrium.
Surface Forces
Nowadays, it is commonly accepted that there are four types of forces in nature: see table below.
Since surface forces scale with object size, they are less appreciable in our everyday lives than they are for instance for insects (water-walking mosquito). In general, surface forces become more and more important the smaller the object. They are thus relevant in technology- and research areas such as colloid science, nano-technology as well as adhesion and nano-tribology. Our interest in surface forces is triggered by fundamental questions related to the dynamic response and structure of solid-fluid interfaces.
Nanotribology and Dynamics of Solid-solid and Solid-fluid Interfaces
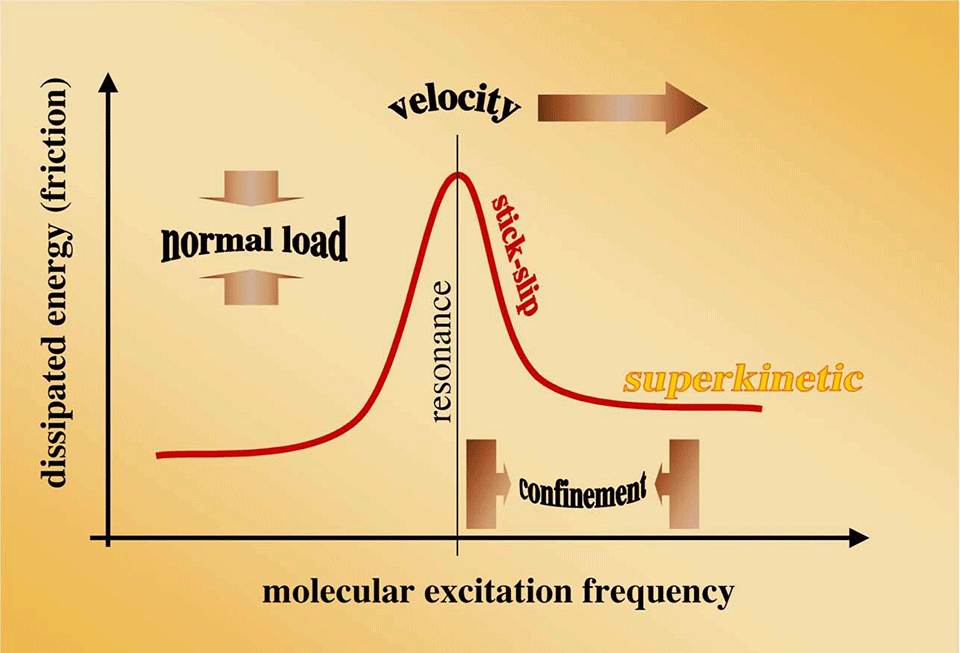
We investigate the fundamentals of dry or boundary-lubricated friction in terms of non-equilibrium processes. At a sliding interface, the equi-partition principle of thermodynamics does not apply; i.e. different degrees of freedom at the interface exhibit different amounts of kinetic energy. There is a net energy flow through the sliding interface. The amount of dissipated energy, dE/dt=F*v, (F: friction force, v: sliding velocity) depends on various dynamic parameters, such as interfacial sliding speed. This project aims at understanding the dependence of interfacial energy dissipation in terms of dynamic processes. In a dynamic process, the dissipated energy strongly depends on the ratio of time scales associated with the local excitation on one hand and the excited degree of freedom on the other. A good model for such a process is the behavior of a damped mechanical oscillator. The amplitude of oscillation and energy dissipation peaks when the system is excited at the resonance frequency. Similar effects are thought to occur locally, on a molecular scale, at the sliding interface. Studying the velocity-dependence is only one way of acting on these phenomena.
Using small-amplitude external excitations, coupled into the sliding interface, we can control the amount of dissipated energy by artificially bringing the interface in a highly excited, superkinetic state. The amount of friction can indeed be controlled without changing the 'usual' parameters such as sliding speed, normal load or temperature, see Picture 3. External excitations can be coupled into the interface in various forms. We are thinking of sub-nanometer mechanical oscillations in-plane or out-of-plane, electric or electromagnetic fields across polar fluids at the interface as well as intentional periodic surface structures. Such intentional surface structures naturally produce local excitations at an interface as a function of structure geometry and interfacial sliding speed. One of the most interesting conclusions is that different modes of interfacial energy dissipation are strictly not additive – rather they can affect each other in a highly non-linear way. While this fundamental non-linearity excludes implementation of an interfacial loss-spectroscopy, it opens doors to dynamic control of dynamic sliding friction.
The extended surface forces apparatus
The surface forces apparatus (SFA) allows one to investigate static and dynamic surface interactions in a well-defined contact geometry. Thin molecular films of fluids, surfactants, boundary lubricants or more complex composition can be confined in a circular single-slit pore. The deflections of compliant springs are used to detect the forces that act in normal and lateral directions through the interface. The SFA-technique has been used since the early seventies and is based on a highly accurate interferometric distance measurement.
Our efforts in the development of the extended surface forces apparatus (eSFA) aim to extend the experimental window of the technique and to automate the technique.
Therefore, the instrumental stability had to be improved by four orders of magnitude.
A spectral correlation method was designed to evaluate interference spectra in a wide spectral range. The precision of thin-film distance measurement was increased 10x-30x, all at a 100x increased distance range. Furthermore, accurate measurement of refractive index was implemented for molecular films down to 1nm film thickness. The dynamic experimental window was extended two orders of magnitude towards the low-speed end and five orders of magnitude towards the high-speed end, see Picture 4.
The eSFA-project is an ongoing effort to increase the instrumental abilities required for fundamental research at solid-fluid interfaces.
Thin-film tribology of chocolate and other complex fluids
In addition to a rheological characterization, friction measurements sensitively provide additional information about the interfacial properties of complex fluids. In collaboration with a chocolate manufacturer we are currently investigating the possible use of this sensitivity for the characterization of molten chocolate samples. A comparison with conventional data from rheology and a sensory panel revealed interesting correlations.
In this context, we are also studying the tribological properties of other complex fluids and model suspensions. The characteristics of the friction trace are found to be strongly dependent on the choice of the substrate materials. Comparative pin-on-disk studies revealed that asymmetric tribo-pairs (two different substrate materials) are not equivalent. A system A/B may thus exhibit considerably different tribological behavior than the analogue B/A system.