PLL-g-PEG Adsorbed on PDMS
An Aqueous-Based Surface Modification of Poly(dimethylsiloxane) with Poly(ethylene glycol) to Prevent Biofouling
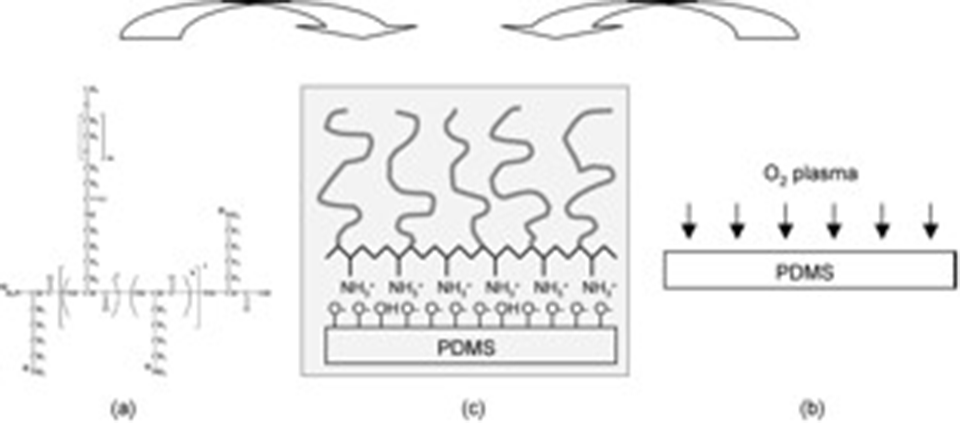
The application of poly(dimethylsiloxane) (PDMS) in biotechnology has been rapidly increasing, e.g., in microfluidic systems and microcontact print. Despite many advantages, the surface properties of PDMS often demand further modification for successful application in biotechnology; nonspecific protein adsorption onto a PDMS surface is one of the most frequently encountered problems, e.g., in microfluidic systems. We have employed a polycation-PEG graft copolymer, poly(L-lysine)-graft-poly(ethylene glycol) (PLL-g-PEG), which directly adsorbs onto the oxidized PDMS surface from aqueous solution. As shown in Figure 1, the polycationic nature of the PLL backbone leads to its electrostatic attraction onto a negatively charged oxidized PDMS surface in an aqueous environment at neutral pH, and thus, a densely packed PEG/water interface can be generated. We can thus avoid organic-solvent-based PEG-ylation approaches, which are known to swell the PDMS network structure.
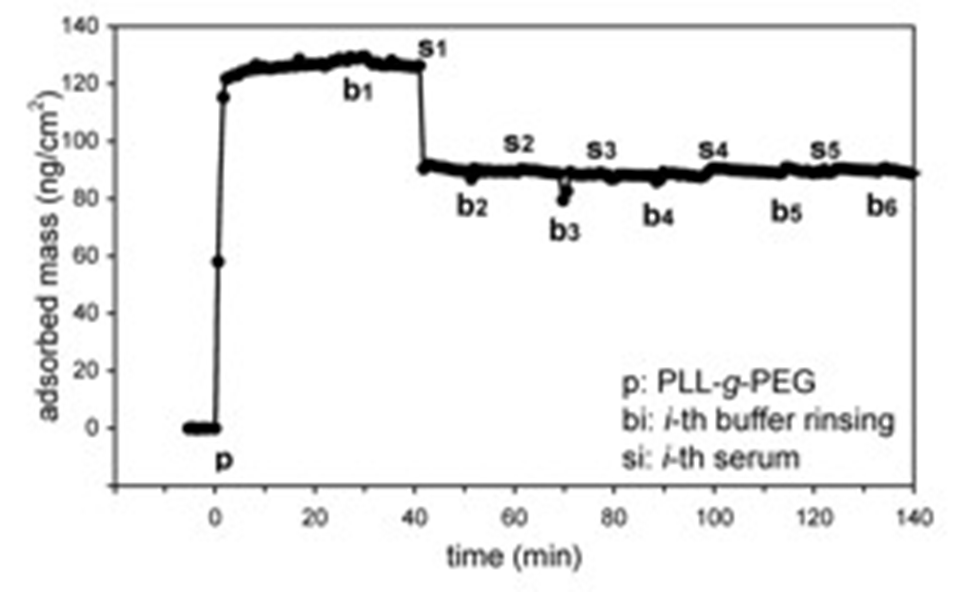
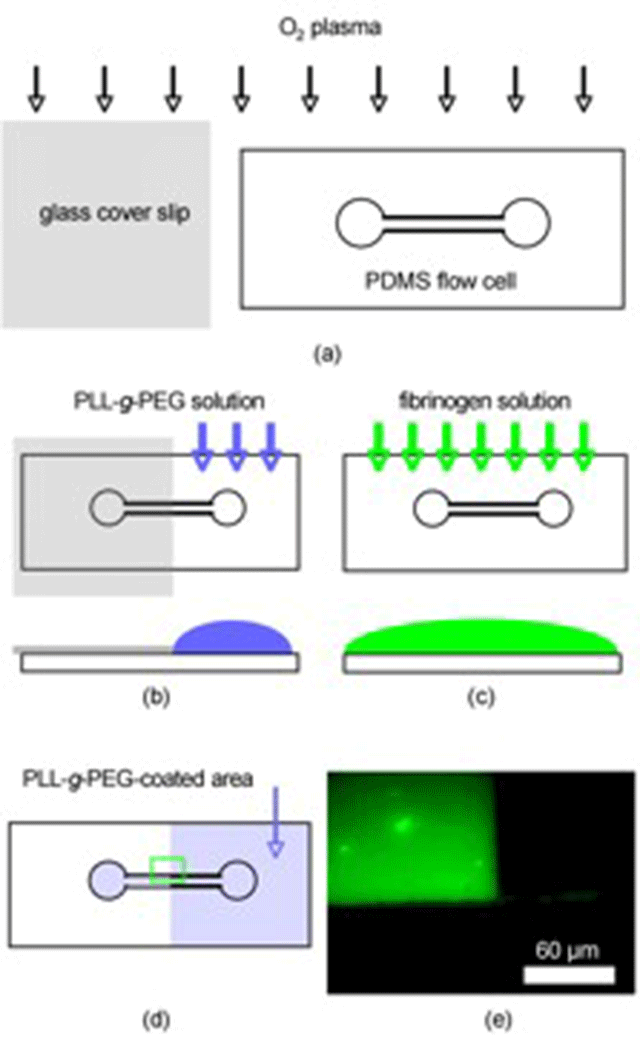
The PEG/aqueous interface generated in this way revealed a near-perfect resistance to nonspecific protein adsorption as monitored by means of optical waveguide lightmode spectroscopy (OWLS) (Figure 2) and Fluorescence Microscopy (Figure 3).