Poly(L-lysine)-g-poly(ethylene glycol)
Poly(L-lysine)-g-poly(ethylene glycol) as a Model Protein-resistant Coating: Mechanical Properties and the Role of Bound Water
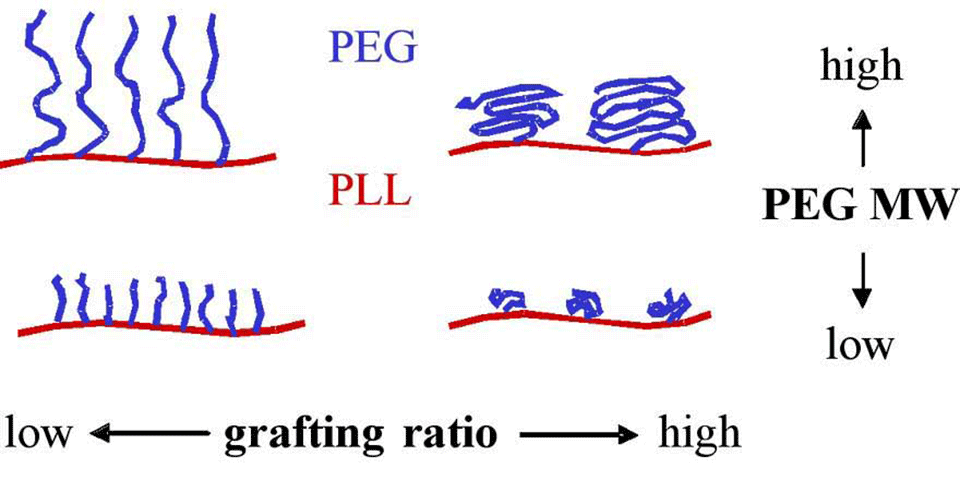
It has been shown that a graft co-polymer of poly(L-lysine) and poly(ethylene glycol), PLL-g-PEG, adsorbed on metal oxide surfaces confers resistance to protein adsorption [1]. Preliminary studies indicated that the degree to which the coated surface resists protein adsorption depends on the co-polymer architecture (molecular weight of PEG chains and the grafting ratio (lysine units/PEG chains)) (Figure 1) [2]. This project is aimed at a systematic investigation of the relationship between the co-polymer architecture, PEG chain conformation, PEG chain hydration, and resistance to protein adsorption.
In the first stage, the synthesis of a polymer matrix enables the co-polymer architecture to be varied in a wide range of molecular weights and grafting ratios. Characterization of the adsorbed polymer with respect to layer thickness, homogeneity and polymer composition is investigated by means of angle-resolved x-ray photoelectron spectroscopy (AR-XPS), spectroscopic ellipsometry (ELM) and time-of-flight secondary ion mass spectrometry (ToF-SIMS). The ability of co-polymers of various architectures to confer resistance to protein adsorption to the metal oxide substrates is evaluated with optical waveguide lightmode spectroscopy (OWLS).
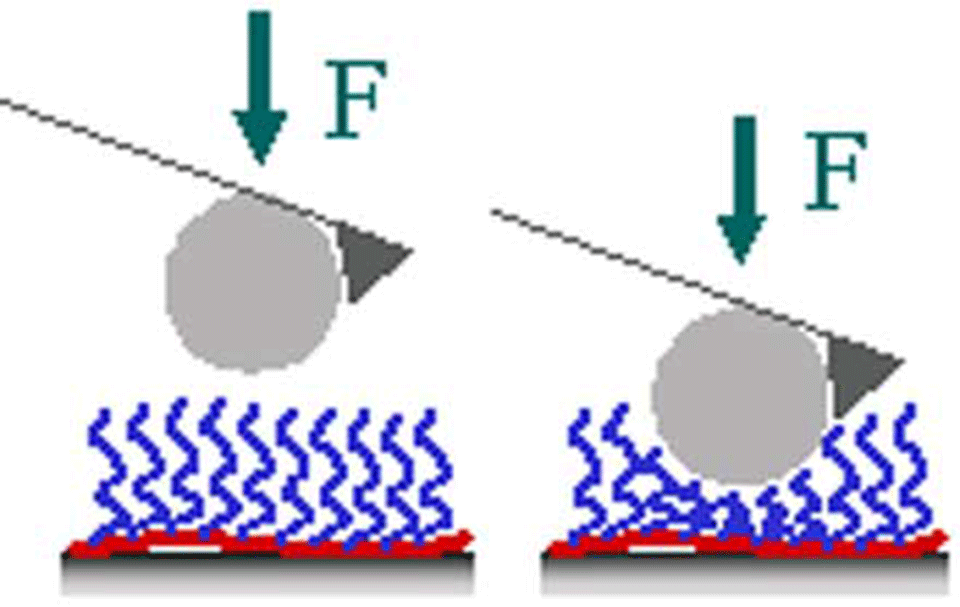
Then, the study of the surface-adsorbed co-polymer by AFM (atomic force microscopy) with a modified silica-tip (Figure 2, 3) provides information about its mechanical properties, which are expected to be correlated with the co-polymer conformation (collaboration with H. J. Griesser, University of South Australia, Adelaide, Australia).
Finally, another AFM technique using modified tips with attached carbon nanotubes (Figure 4) allows elucidating the role of hydration of PEG chains in resistance to protein adsorption and possibly PEG chains dynamics (collaboration with S. Jarvis, Trinity College, Dublin, Ireland).
Relating the conformation and the hydration of PEG chains to the architecture of the co-polymer on one hand and to the resistance to protein adsorption on the other is expected to lead to the elaboration of a model and to provide the knowledge necessary for the design of other protein-resistant coatings.
References
- Elbert, D.L., “Self-assembly and steric stabilization at heterogeneous, biological surfaces using adsorbing block copolymers”, Chemistry and Biology 1998, 5 (3), 177-183
- Kenausis, G.L. et al., “Poly(L-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: Attachment mechanism and effects of polymer architecture on resistance to protein adsorption”, J. Phys. Chem. B 2000, 104, 3298-3309.
Partners
- external pageProf. H.J. Griesser, University of South Australia, Adelaide, Australiacall_made
- Prof. S.P. Jarvis, Trinity College, Dublin, Ireland
Funding
- National fund, ETHZ and EPFL

![Enlarged view: Figure 2: Scanning electron microscopy image of a silica microsphere glued to a standard AFM tip [H. Griesser]](/research/surface-functionalization/functionalization-with-grafted-polyelectrolytes/poly-l-lysine-g-poly-ethylene-glycol/_jcr_content/par/fullwidthimage_503200301/image.imageformat.1286.1004206672.png)