Functionalization with Phosphates
Alkane Phosphate Self-Assembled Monolayers (SAM): Modification of Metal Oxide Surfaces for Controlled Physico-Chemical Properties
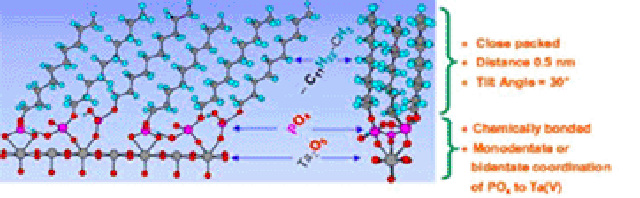
Self-assembled monolayers (SAMs) of thiols on gold surfaces are widely used to produce model surfaces with well-defined chemical composition for a variety of applications including biomaterial and biosensor surfaces [1,2]. Alkane-phosphates and -phosphonates have been shown to form SAMs on a number of transition metal oxide surfaces such as titanium oxide (TiO2), tantalum oxide (Ta2O5) and niobium oxide (Nb2O5) [3,4]. Using a combination of dedicated surface characterization techniques, the molecular structure of these adlayers has been demonstrated to be similar to thiols on gold with an intermolecular spacing of 5 Å (corresponding to 21 Å2 per molecule) and a tilt angle of the molecular axis of 30-35° relative to the surface normal (Figure 1). The binding of the phosphate head group to the metal oxide surface is believed to be through direct coordination between phosphate and metal cation and arguments have been given for the presence of both mono- and bi-dentate binding of the phosphate group thus enabling the formation of a close-packed layer.
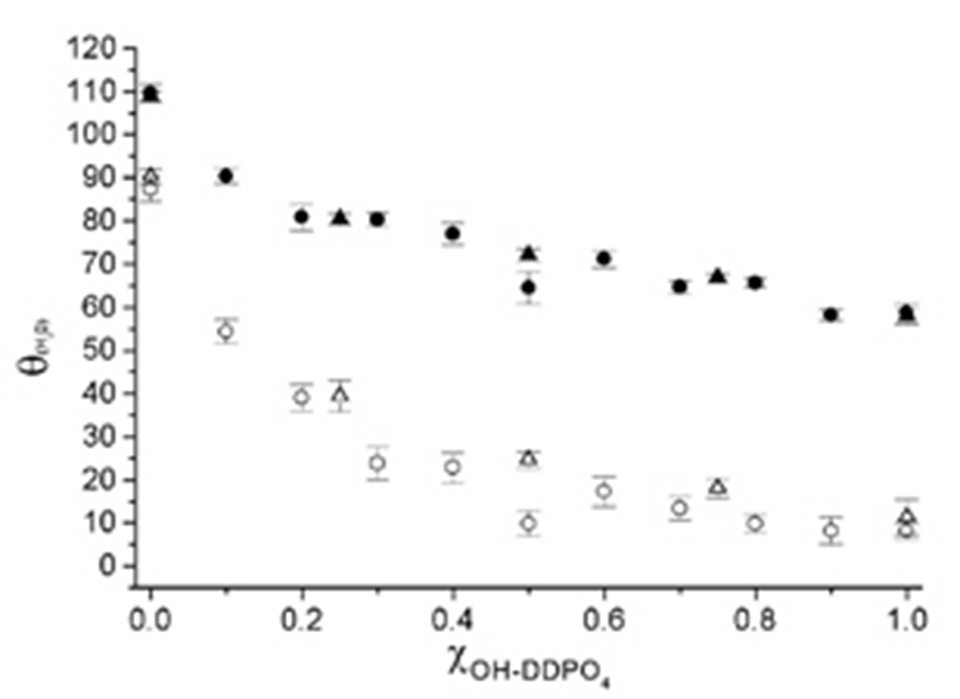
Functional groups placed opposite to the phosph(on)ate group allow tailoring of surface properties (e.g. hydrophilicity/electrical charge/protein resistance or specific protein adsorption) as shown in Figure 2, were the advancing and receeding contact angles (measure for the wettability of a surface) of smooth titanium oxide surfaces after formation of a self-assembled monolayer from mixed aqueous solution of DDPO4/OH-DDPO4 is showed [5]. The contact angles depend the surface chemistry (composition of SAM) of the surface.
References
- Bain, C. D. et al., J. Am. Chem. Soc. 111, 321 (1989).
- Nuzzo, R.; Allara, D. L., J. Am. Chem. Soc. 105, 4481-4483 (1983).
- Brovelli, D. et al., "Highly Oriented, Self-Assembled Alkanephosphate Monolayers on Tantalum(V) Oxide Surfaces", Langmuir 15, 4324-4327 (1999).
- Textor, M. et al., "Structural Chemistry of Self-Assembled Monolayers of Octadecylphosphoric Acid on Tantalum Oxide Surfaces", Langmuir 16, 3257-3271 (2000).
- Tosatti, S. et al., "Self-Assembled Monolayers of Dodecyl and Hydroxy-Dodecyl Phosphate on both Smooth and Rough Titanium and Titanium Oxide Surfaces", Langmuir (2002).
Related Subjects
- Self-Assembled Monolayers for Titanium Implants
- Surface Micro- and Nanopatterning