In Situ ATR Tribometry
Introduction
In order to meet the very stringent emission requirements that will be introduced over the next few years, zinc-, phosphorus- and sulfur-containing additives, currently used in engine oil formulations, must be replaced by alternative, ecologically compatible additives. A better understanding of the mechanism of tribochemical reactions on the surfaces of lubricated systems under tribological conditions is necessary, in order to design new additives with greater environmental compatibility, while not compromising their performance.
ATR FT-IR Tribometer
The main objective of this project is the development of a new technique for in situ studies of tribological systems, involving Attenuated Total Reflection Infrared (ATR FT-IR) spectroscopy. The ATR FT-IR analysis is combined with tribological experiments, in order to probe the lubricant layer between sliding surfaces by measuring ATR spectra from the underside of a thin metallic film. In this way, the metal surface/lubricant interface can be continuously monitored, in situ, and in a non-destructive manner during a tribological sliding experiment.
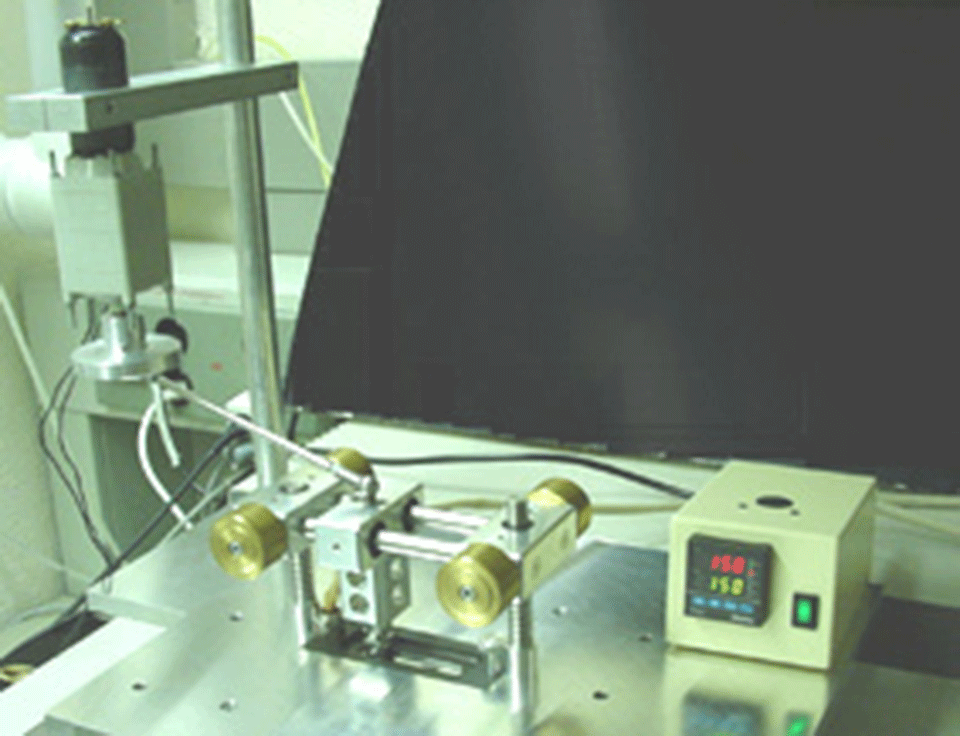
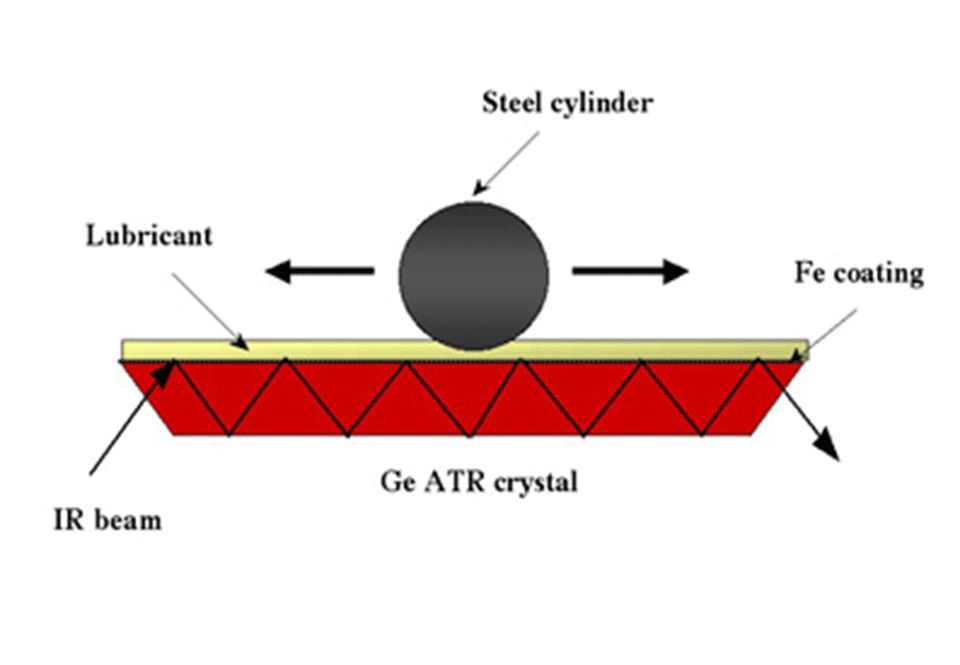
ATR tribological tests are performed with a tribometer in which a fixed steel cylinder slides against an iron-coated germanium ATR crystal (Figure 1).
Figure 1: Photograph (Figure 1.1) and schematic (Figure 1.2) of the ATR FT-IR Tribometer for the in situ chemical analysis of tribological films.
Tribochemistry of zinc dialkyldithiophosphates (ZnDTP)
The current work focuses on the application of in situ ATR FT-IR Tribometry to the study of tribofilms formed from solutions of organophosphate additives (ZnDTP) in poly-a-olefin (PAO) lubricant on an iron-coated germanium ATR crystal. Changes in the ATR FT-IR spectrum of the near-surface region due to thermal and/or tribo-chemical reactions occurring between the lubricant additive and the iron surface have been investigated as a function of time.
The ATR results on the surface reaction of ZnDTP under purely thermal conditions indicate the formation of P-O-P bonds, characteristic of polyphosphates (Figure 2).
The modification of the ZnDTP molecule with the formation of (POO)- species, detected after friction at 150°C (Figure 3), could be an intermediate stage in the formation of inorganic phosphate.
Relevance of in situ ATR FT-IR Tribometry to the field of tribochemistry
ATR Tribometry, as an in situ analytical technique, has the advantage that the ATR spectra of growing thermal/or tribological films can be measured as they are formed, under steady-state conditions, through a thin metallic film representing one of the rubbing surfaces.
The in situ ATR analysis allows tribological conditions, such as normal load and velocity, to be directly correlated with changes in the chemistry of the lubricant/metal surface interface.
In the case of the ZnDTP lubricant additives, the in situ ATR Tribometry allows the kinetics of formation of tribofilms and the relative roles of tribochemical and thermochemical processes to be investigated.
References
- F.Piras, A. Rossi, N. D. Spencer, In-situ attenuated total reflection (ATR), spectroscopic analysis of tribological phenomena, Trib. Ser.40, "Boundary and Mixed Lubrication: Science and Application", 199-206, 2002
- F. Piras, A. Rossi, N.D. Spencer, The growth of tribological films: in situ characterization based on Attenuated Total Reflection Infrared Spectroscopy, Langmuir, 18, 6606-6613, 2002
- F. M. Piras, A. Rossi, N. D. Spencer, Combined in-situ (FT-IR) and ex-situ (XPS) study of ZnDTP - iron surface interaction, Tribology Letters, 15 (3), 181 - 191, 2003.